Which Best Describes Teh Ions in Neutral Solutions
H acceptor B acidic. Which of the following best describes this solution.
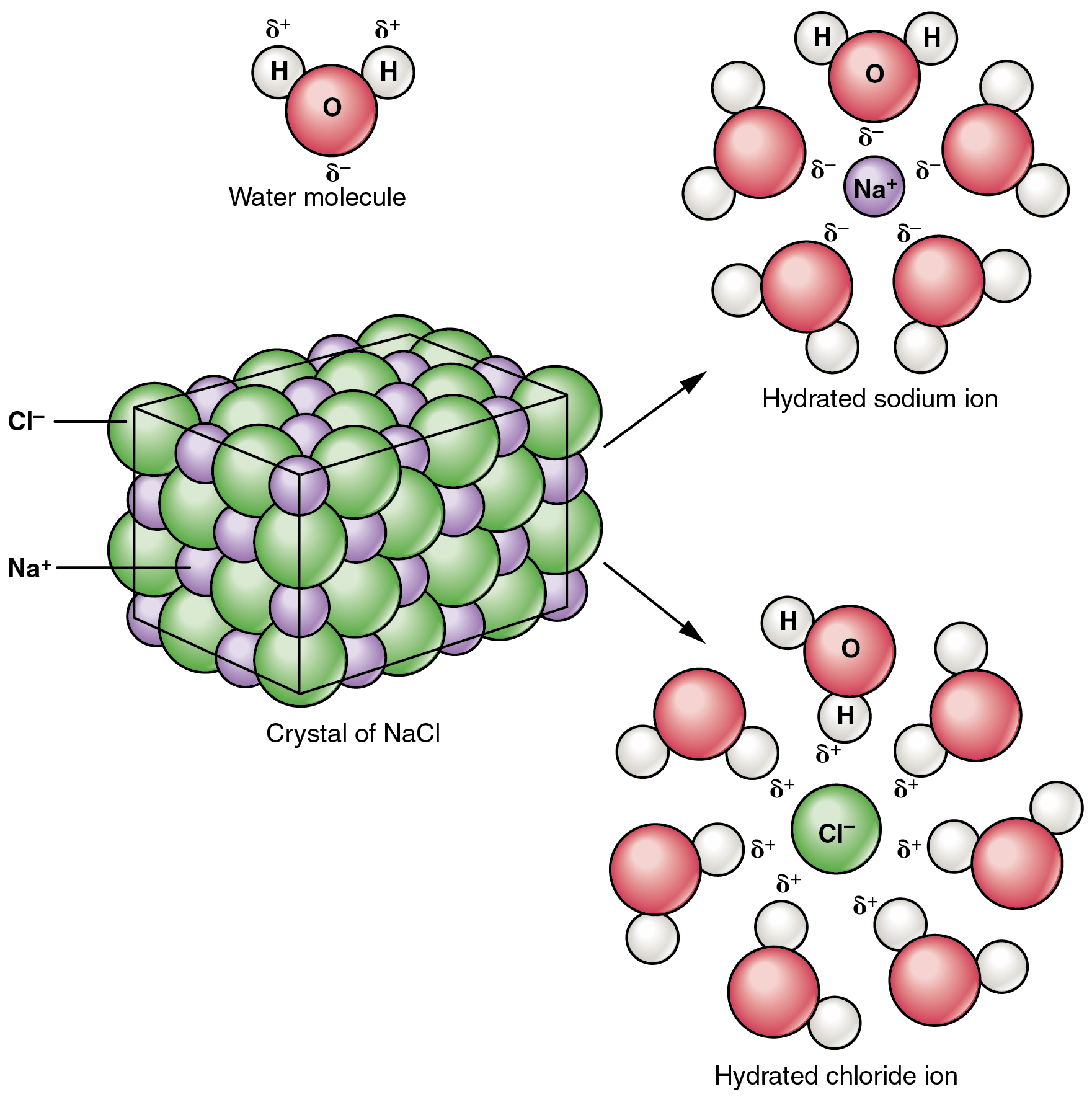
Molecular Complete Ionic And Net Ionic Equations Article Khan Academy
Biology questions and answers.

. Acids measure below 7. A solution contains 00000001 10-7 moles of hydrogen ions H per liter. Which of the following best describes aqueous sulfuric acid H 2 SO 4 aq in the lead-acid battery.
A In aqueous solution sulfuric acid exists almost entirely as the neutral H 2 SO 4 molecule. 2 POINTS A acidic. 5 A solution contains 10-3 moles of hydroxyl ions OH- per liter.
Which would be least likely to completely dissolve in water. H donor D basic. Molecule and the HSO 4.
D Water molecules exert forces of attraction that break the potassium chloride apart into potassium atoms and diatomic chlorine gas. H acceptor B basic. Calculate the pH of a solution in which OH71103M.
As you saw in the video the pH of a 01 M solution of acetic acid HC2H3O2 Ka 18 105 is 29. A solution contains 10-3 moles of hydroxyl ions OH- per liter. A solution has a pH of 8.
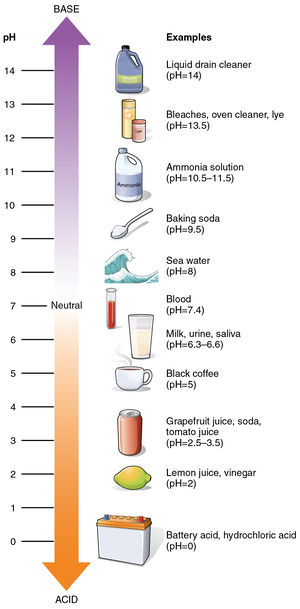
Which best describes the pH scale. It has a pH of 2 and is a base. Which best describes the solutions.
32 A solution contains 00000001 10-7 moles of hydroxyl ions OH- per liter. Indicate whether the solutions in Parts A and B are acidic or basic. H acceptor C acidic.
What do hydrogen ions. Which of the following best describes this solution. Ht acceptor B basic.
Which best describes the solution. A student prepares a 110-8 M solution of HCl. Respiring cells release CO2.
C Water molecules exert forces of attraction that break the potassium chloride apart into neutral atoms of potassium and neutral atoms of chlorine. Which best describes the. Which is the most likely charge of the ion.
What is the pH. Which of the following best describes this solution. How does the addition of an acid affect a neutral solution the h ion concentration is increased a given solution contains 000011 10-4 moles of hydrogen ions h per liter.
Solution A is more acidic than solution B. Carbon dioxide CO2 is readily soluble in water according to the equation CO2 H2O H2CO3. H acceptor C acidic.
Carbonic acid H2CO3 is a weak acid. A neutral atom of a certain element has the electron configuration 1s 2 2s 2 2p 6 3s 2 3p 4. Acidification would decrease dissolved carbonate concentrations and hinder growth of corals and shell-building animals.
Which best describes the solution. An antacid decreases the level of hydrogen ions. H acceptor C acidic.
Which of the following best describes this solution. An antacid increases the amount of hydrogen ions. How many valence electrons does the atom have.
H donor D neutral Answer. Which of the following best describes this solution. A solution has a pH of 2.
Which of the following best describes this solution. A solution contains 00000001 10-7 moles of hydroxyl ions OH- per liter. Which best describes the solution.
Is the solution acidic neutral or basic. The neutral atom becomes an ion during a chemical reaction. H donor E neutral.
An antacid neutralizes excess hydrogen ions. If the H 10-8M Then poH - log 10-8 pH 800 pH 700 solution is basic. A solution has a pH of2.
H donor D neutral. Now perhaps you would like to try answering this question. Arrhenius acid act as a good electrolyte as it dissociates to its respective ions in the aqueous solutions.
Keeping it similar to the general acid properties Arrhenius acid also neutralizes bases and turns litmus paper into. A point a strong acid. Apart into potassium ions and chloride ions.
B In aqueous solution sulfuric acid exists as a mixture of significant concentrations of both the neutral H 2 SO 4. Polar QUESTION 3 A solution contains 0000000110-7 moles of hydroxyl ions OH- per liter. Which statement best describes an antacid.
5 A solution contains 10-3 moles of hydroxyl ions OH- per liter. Which of the following best describes this solution. H acceptor QUESTION 4 In pure water H and OH- concentrations are equal.

Why Ph Value Is Measured Between 0 And 14 Pharmaceutical Guidelines

Let S Talk Science A Ph Solution Washington State Department Of Ecology

Crash Course World History Literature Us History Chemistry Ecology Biology Chemistry Lessons Teaching Chemistry Crash Course
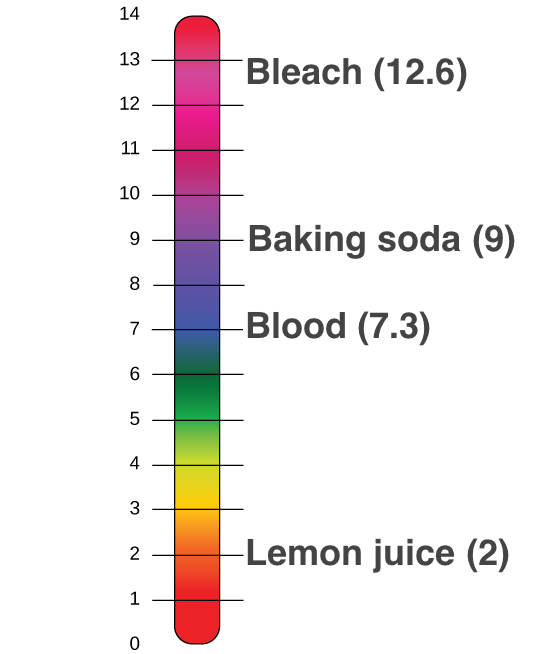
Ph Acids And Bases Review Article Khan Academy

Introduction To Ph Video Khan Academy

Benzaldehyde Structure Chemistry Notes Chemistry Formula

Acid Base Properties Of Salts Video Khan Academy

Neutral Solution Definition Examples Video Lesson Transcript Study Com

Acids And Bases Ck 12 Foundation
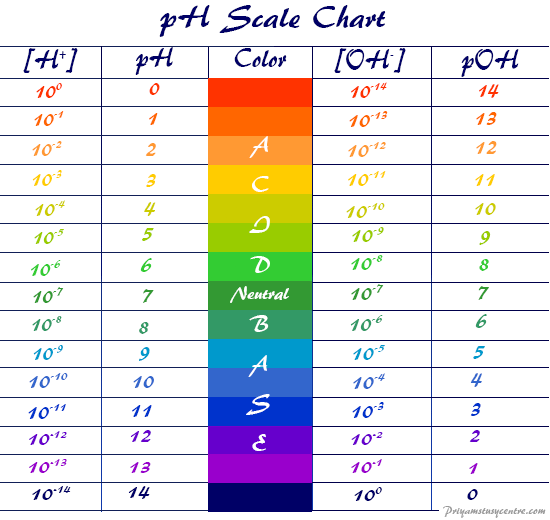
Ph Scale Poh Scale Definition Range Chart Measurement

Acids And Bases I Introduction
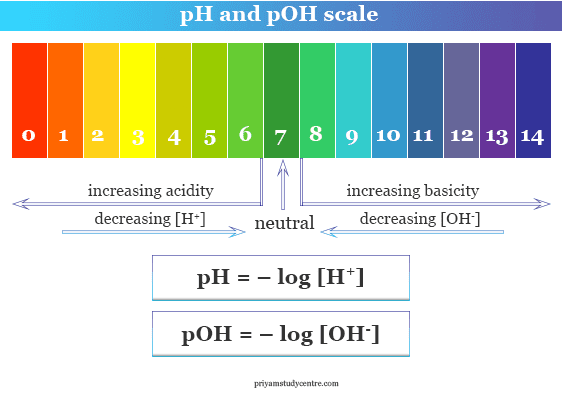
Ph Scale Poh Scale Definition Range Chart Measurement

Pinterest The World S Catalog Of Ideas

Traces Of Ancient Life Tell Story Of Early Diversity In Marine Ecosystems Study School Of Medicine Analysis

16 6 Finding The H3o And Ph Of Strong And Weak Acid Solutions Chemistry Libretexts

Avogadro S Number And The Mole Chemistry Education Chemistry Lessons Teaching Chemistry

How To Calculate The Concentration Of A Solution Chemistry Lessons Chemistry Education Teaching Chemistry

Comments
Post a Comment